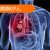
| 効果 | 増加 |
| 試験方法 | コホート法 |
| 試験期間 | 2-4 週間 |
| 被験者数 | 82 |
| 性別 | 男女 |
| 年齢 | 30-44, 45-64 |
| 体型 | 肥満 |
200mg of rhodiola extract (300-1000mg root equivalent) twice daily for 4 weeks in persons with life and work-related stress was greatly able to reduce dysfunction and fatigue associated with stress in a time-dependent manner. Significant improvements were noted in social and work function secondary to reduced fatigue and improved mood.
A Double-blind, Placebo-controlled Pilot Study Of The Stimulating And Adaptogenic Effect Of Rhodiola Rosea SHR-5 Extract On The Fatigue Of Students Caused By Stress During An Examination Period With A Repeated Low-dose Regimen
| 効果 | 増加 |
| 試験方法 | 二重盲検法 |
| 試験期間 | 2-4 週間 |
| 被験者数 | 40 |
| 性別 | 男性 |
| 年齢 | 13-17, 18-29 |
| 体型 | 平均 |
20 days of rhodiola supplementation during examination periods for students (100mg SHR-5) was able to improve neuromotoric fitness (accuracy of maze drawing test), fatigue, and well being relative to placebo. Exam scores were 8.4% higher in the rhodiola group relative to placebo.
A Randomized Trial Of Two Different Doses Of A SHR-5 Rhodiola Rosea Extract Versus Placebo And Control Of Capacity For Mental Work
| 効果 | 増加 |
| 試験方法 | 二重盲検法 |
| 試験期間 | 1-7 Days |
| 被験者数 | 121 |
| 性別 | 男性 |
| 年齢 | 18-29 |
| 体型 | トレーニング中, 肥満, 平均 |
In military cadets performing regular military night duties, 5 days of supplementation of rhodiola (370 or 555mg of SHR-5) was able to significantly reduce total fatigue and improve well being when measured 2 hours after supplement ingestion and again at the end of the trial. Capacity for mental work significantly increased relative to placebo.
Clinical Trial Of Rhodiola Rosea L. Extract SHR-5 In The Treatment Of Mild To Moderate Depression
| 効果 | 増加 |
| 試験方法 | 二重盲検法 |
| 試験期間 | 1-6 ヶ月 |
| 被験者数 | 89 |
| 性別 | 男女 |
| 年齢 | 18-29, 30-44, 45-64 |
42 days supplementation of 340-680mg rhodiola SHR-5 extract was able to significantly reduce the symptoms of depression in persons with diagnosed depression, with only partial dose dependence noted.
The degree of improvement ranged from 30-35% (HAMD rating scale) to 50% reduced symptoms (higher dose on BDI).